Introduction:
Induction with pediatric-inspired regimens (PIR) in young adults with acute lymphoblastic leukemia (ALL) is being increasingly used due to its demonstrated improvement in overall survival (OS) compared to historical adult regimens. However, in certain comparisons, PIR, specifically augmented BFM, did not exhibit an OS benefit when compared to the adult Hyper-CVAD regimen. Additionally, Hispanic patients with ALL often present with disease-related high-risk features and a high prevalence of comorbidities like obesity and metabolic syndrome, which may contribute to unfavorable outcomes and a particular adverse event profile. This study aimed to compare the OS and relapse-free survival (RFS) of patients treated with a PIR, a modified CALGB 10403 (mCALGB), and the Hyper-CVAD regimen.
Methods:
This observational retrospective cohort study was conducted at a single reference center in Mexico City. The study included adult patients aged 18-49 years with newly diagnosed Philadelphia (Ph)-negative ALL who received induction chemotherapy with either mCALGB or Hyper-CVAD between January 2015 and May 2023. High-risk karyotype was defined by hypodiploid, complex, or t(v;11q23), and hyperleukocytosis was characterized by > 30 x 10 3/µL white blood cells. Measurable residual disease (MRD) was assessed via flow cytometry. A comparative analysis of sociodemographic, clinical, and laboratory characteristics was performed between the two treatment groups using Student's t-test (parametric) or U Mann-Whitney (non-parametric) for continuous variables, and the Chi-square / exact Fisher tests for categorical variables. OS and RFS were estimated using Kaplan-Meier survival analysis, and the log-rank test was used to compare both survivals. A Cox regression analysis was performed to identify prognostic factors for OS and RFS.
Results:
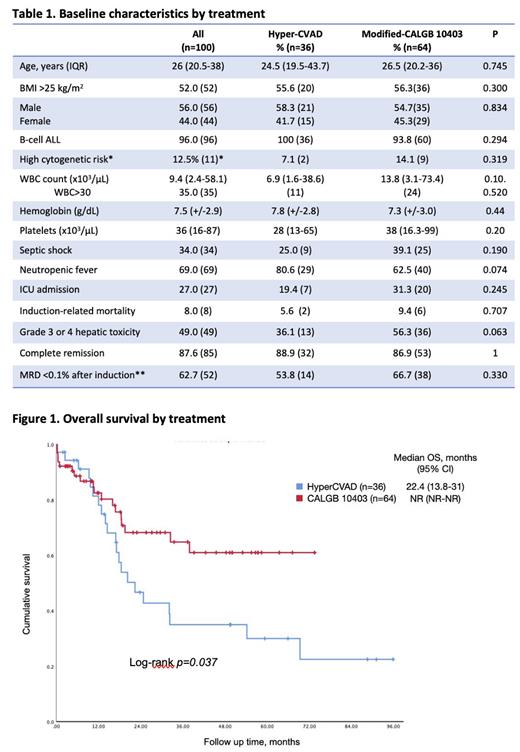
The study included 100 patients: 36 treated with Hyper-CVAD and 64 with mCALGB. The median age at diagnosis was 26 years (IQR 20.5-38), with 56% being male, and 52% being overweight or obese. No statistically significant differences were observed in the clinical and laboratory features between the treatment groups (see Table 1).
Regarding induction-related mortality, febrile neutropenia, or admission to an intensive care unit during induction, no differences were found between Hyper-CVAD and mCALGB (see Table 1). A non-significant trend towards a higher rate of grade 3 or 4 hepatotoxicity during induction was observed in patients receiving the modified CALGB 10403: 56.3% vs. 36.1% with an odds ratio (OR) of 2.275 (95% CI 0.982-5.272), p=0.063.
The complete response (CR) rate was 87.6%. MRD was assessed in 83% of patients, with <0.1% detected in 62.7% of them. There were no differences between Hyper-CVAD and mCALGB regarding CR or MRD<0.1% (88.9% vs. 86.9%, p=1, and 53.8% vs. 66.7%, p=0.330, respectively).
The relapse rate for the entire population was 41.8%, with Hyper-CVAD demonstrating a higher relapse rate compared to mCALGB: 64.7% vs. 28.1%, p=0.001. The median RFS was 54.1 months (95% CI NR-NR) for mCALGB and 12.7 months (95% CI 2.9-22.5) for Hyper-CVAD, with a 3-year RFS of 61.2% vs. 31.5%, p=0.008. In a multivariate analysis for RFS, significant prognostic factors included WBC >30 x 10 3/µL (HR 4.47, 95% CI 2.09-9.57, p<0.001), post-induction MRD >0.1% (HR 4.07, 95% CI 1.86-8.90, p<0.001), and mCALGB vs. Hyper-CVAD (HR 0.37, 95% CI 0.18-0.78, p=0.01).
The median OS for the entire cohort was 32.3 months (95% CI 28.3-80.1), with mCALGB demonstrating a non-reached (NR) median OS (95% CI NR-NR), and Hyper-CVAD exhibiting a median OS of 22.4 months (95% CI 13.8-31) with a 3-year OS of 64.8% vs. 35.0%, p=0.037 (see Figure 1). In a multivariate analysis for OS, significant prognostic factors included WBC >30 x 10 3/µL (HR 3.57, 95% CI 1.58-8.09, p=0.002), post-induction MRD >0.1% (HR 2.58, 95% CI 1.17-5.69, p=0.002), and mCALGB vs. Hyper-CVAD (HR 0.29, 95% CI 0.13-0.66, p=0.003).
Conclusion: Among a cohort of Mexican patients, treatment with mCALGB was independently associated with better RFS and OS compared to Hyper-CVAD.
Disclosures
Demichelis:Pfizer: Consultancy, Honoraria; TEVA: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; AMGEN: Consultancy, Honoraria; Abbvie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal